It is of the utmost importance that you take care of your heart. A healthy heart is the key to a long, healthy life. However, some people need a little assistance in ensuring that their heart is functioning correctly.
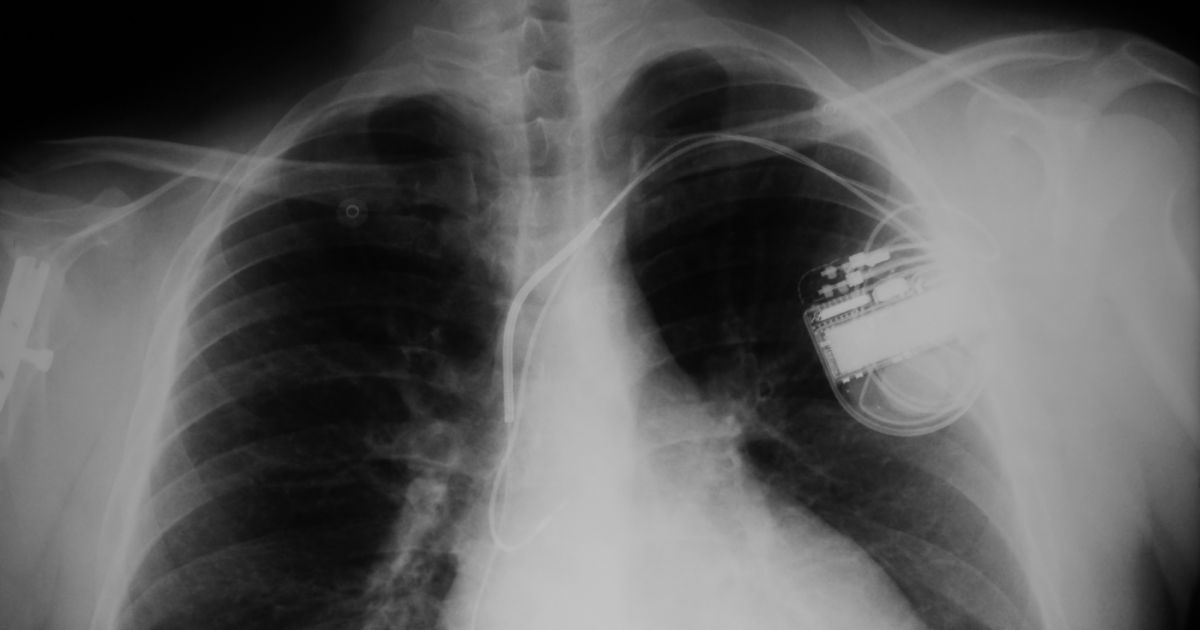
Having a heart pump surgically implanted is one way to get medical assistance. For some, that assistance has led to problems. The U.S. Food and Drug Administration (FDA) recently announced that Medtronic had recalled more than 1,600 HVAD pump kits. The HVAD pump has already been linked to the death of one patient, with two more patients injured. Unfortunately, for many patients with the pump already in them, removing this potentially dangerous device could present more complications than keeping it in.
In the announcement, the recall is listed as Class I, the most serious type of recall. This is due to the possibility of the HVAD pump causing serious injury or death due to a malfunction found after Medtronic received numerous complaints from patients.
This recall was not the first for Medtronic. It had already recalled numerous HVAD pump kits before the Class I recall was announced early in June. A few deaths were also reported that were linked to that recall.
The recent HVAD pump kit recall announced by the FDA has brought light to a potentially severe problem for many people.
The HVAD pump is surgically implanted around the pericardial space, a sac that surrounds the heart. The HVAD connects to the bottom of the left ventricle, drawing oxygen-rich blood through the pump and into the aorta, a large blood vessel that allows blood to flow throughout the body.
The HVAD pump is for patients at risk for end-stage left ventricular heart failure, for those who need heart tissue recovery, or it is used as destination therapy. It is essential to understand that a person could already have heart failure but not know it. Having heart failure does not necessarily mean that the heart has completely shut down, it might be that the heart is weak and in the process of failing.
Telltale signs of heart failure are shortness of breath and fatigue. Normal everyday activities become a chore, such as walking, climbing stairs, and lifting. The heart will not get better on its own.
Heart failure can manifest in many ways. For instance, it can affect the kidneys, limiting their capacity to dispose of waste. Eventually, heart failure causes the heart to become so weak that it simply cannot pump enough blood to meet the body’s needs.
According to the patient manual for the HVAD system, the pump is for patients with heart failure who have not shown improvement from other treatments. Sometimes, patients may have the pump implanted while waiting for a heart implant. In other cases, the pump may be used in patients who need destination therapy.
After receiving complaints from patients experiencing pump thrombosis symptoms, Medtronic took some of those patients’ removed pumps and examined them. The examinations resulted in the finding of a welding defect. The pumps were allowing moisture to leak into the core, which caused the internal magnets to become corroded and demagnetized. The pumps, subsequently, begin rotating improperly.
According to the FDA, the HVAD pump could delay the process of restarting or stop altogether, increasing the risk of serious injury or death. Injuries from the device may include shock, severe organ dysfunction, or stroke.
According to the FDA, a letter from Medtronic was sent in April to the appropriate physicians and health care providers, urging them not to assign any unused pump implant kits for surgery and that they should immediately return them.
Later, Medtronic sent another letter to the same medical facilities to advise them that the HVAD pump kits in their possession, including ones already implanted in patients, could possibly have a welding defect.
In the letter, Medtronic listed possible symptoms that patients who already have the device might incur. If a patient has any of the listed symptoms, the health care provider should submit those findings to Medtronic and proceed accordingly. Symptoms may include abnormal pump sounds, low perfusion, which means that blood flow is moving at an insufficient speed, lightheadedness, and dizziness.
Medtronic is urging physicians to treat each case according to the specific symptoms and health of the patient. The physician should establish whether the symptoms show signs of being caused by pump thrombosis. This may determine how the physician is to proceed and whether surgery is the best option. Considering surgical risks for each patient is critical.
With the FDA working closely with Medtronic, a support program has been set up for patients who already had the HVAD implanted. The FDA has clarified that it is a priority for patients to receive the best medical care possible. The road ahead may be difficult for patients experiencing symptoms caused by a defective HVAD pump. However, they may be able to file a medical products liability claim with help from a lawyer.
A faulty surgical implant could cause pain and suffering or death. If you have a defective implant or any other medical product, one of our experienced Philadelphia medical products liability lawyers at Brookman, Rosenberg, Brown & Sandler will fight on your behalf. Call us at 215-569-4000 or contact us online for a free consultation. Located in Philadelphia, we serve clients throughout New Jersey and Pennsylvania, including Delaware County, Chester County, and Philadelphia County.